A radioactive substance decays exponentially, embarking on a fascinating journey where its radioactivity diminishes over time, governed by the laws of exponential decay. This concept holds profound implications in fields ranging from medicine to archaeology, offering insights into the nature of matter and the passage of time.
Radioactive decay, characterized by the emission of particles or energy from unstable atomic nuclei, follows an exponential pattern. The rate of decay is proportional to the amount of radioactive material present, leading to a gradual decrease in radioactivity over time.
Radioactive Decay
Radioactive decay is a process in which an unstable atomic nucleus loses energy by emitting radiation, resulting in the transformation of the atom into a more stable atom. This process is accompanied by the release of energy in the form of alpha, beta, or gamma radiation.
Examples of radioactive elements include uranium, thorium, and plutonium. These elements have unstable nuclei that undergo radioactive decay over time.
The rate of radioactive decay is affected by several factors, including the type of radioactive element, the temperature, and the presence of a catalyst.
Exponential Decay
Exponential decay is a mathematical function that describes the decrease in the amount of a substance over time. The mathematical equation for exponential decay is:
$$N(t) = N_0e^-kt$$
where:
- $$N(t)$$ is the amount of the substance at time $$t$$
- $$N_0$$ is the initial amount of the substance
- $$k$$ is the decay constant
In the case of radioactive decay, the decay constant is a measure of the probability that a radioactive atom will decay per unit time.
Half-Life: A Radioactive Substance Decays Exponentially

Half-life is the time it takes for half of the radioactive atoms in a sample to decay. The half-life is related to the decay constant by the following equation:
$$t_1/2 = \frac\ln 2k$$
where:
- $$t_1/2$$ is the half-life
- $$k$$ is the decay constant
Examples of half-lives for different radioactive elements include:
- Uranium-238: 4.47 billion years
- Thorium-232: 14.05 billion years
- Plutonium-239: 24,100 years
Applications of Radioactive Decay
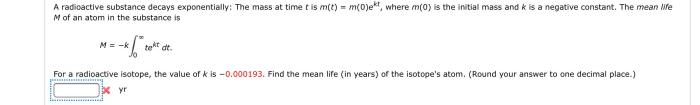
Radioactive decay has a wide range of applications in various fields, including:
Medicine, A radioactive substance decays exponentially
- Radioactive isotopes are used in medical imaging techniques such as X-rays and PET scans.
- Radiotherapy uses radioactive isotopes to kill cancer cells.
Dating Techniques
- Radioactive decay is used in radiometric dating to determine the age of rocks, fossils, and other objects.
Industrial Applications
- Radioactive isotopes are used in smoke detectors and ionization chambers.
- Radioactive isotopes are used in food irradiation to preserve food.
Health Effects of Radioactive Substances

Exposure to radioactive substances can have adverse health effects, including:
- Radiation sickness
- Cancer
- Birth defects
The different types of radiation and their effects on human health include:
- Alpha radiation: Alpha particles are large and have a short range, but they can cause significant damage to cells if they are ingested or inhaled.
- Beta radiation: Beta particles are smaller and have a longer range than alpha particles, but they are less damaging to cells.
- Gamma radiation: Gamma rays are electromagnetic waves with a very high energy and a long range, and they can penetrate the body and damage cells.
Guidelines for safe handling and disposal of radioactive substances include:
- Use proper shielding to protect against radiation exposure.
- Handle radioactive materials in a well-ventilated area.
- Dispose of radioactive waste in accordance with local regulations.
FAQ Section
What is radioactive decay?
Radioactive decay is the process by which unstable atomic nuclei emit particles or energy, transforming into more stable nuclei.
How does exponential decay relate to radioactive decay?
Exponential decay describes the pattern of radioactive decay, where the rate of decay is proportional to the amount of radioactive material present.
What is half-life?
Half-life is the time it takes for half of a radioactive sample to decay.