Enzymes and cellular regulation pogil play a crucial role in the intricate workings of living organisms. Enzymes, the catalysts of biochemical reactions, orchestrate a vast array of cellular processes, ensuring the smooth functioning of life. This comprehensive guide delves into the fundamentals of enzymes, their regulation, and their significance in cellular metabolism.
Enzymes are intricate molecular machines, each tailored to a specific biochemical reaction. Their structure and composition determine their catalytic abilities, enabling them to accelerate reactions by lowering activation energy. Understanding enzyme activity and regulation is essential for comprehending cellular metabolism and the intricate interplay of biological systems.
Enzyme Fundamentals
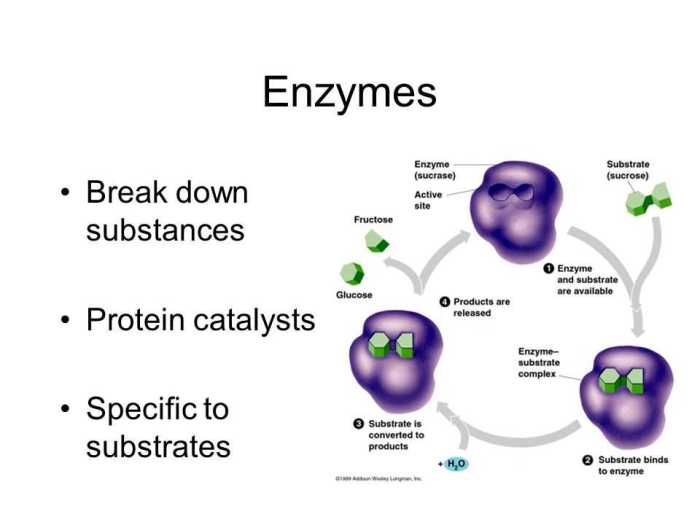
Enzymes are biological catalysts that enhance the rate of chemical reactions within cells. They are protein molecules with a specific three-dimensional structure that enables them to bind to specific substrates, the molecules they act upon.
Enzymes play a crucial role in cellular processes by facilitating reactions that would otherwise occur too slowly to sustain life. They are essential for metabolism, energy production, DNA replication, and numerous other cellular functions.
Types of Enzymes
Enzymes are classified based on the type of reaction they catalyze. Some common types include:
- Oxidoreductases:Transfer electrons between molecules.
- Transferases:Transfer functional groups between molecules.
- Hydrolases:Break down molecules by adding water.
- Lyases:Break down molecules by removing groups without hydrolysis.
- Isomerases:Convert molecules into isomers (molecules with the same formula but different structures).
- Ligases:Join two molecules together, often with the hydrolysis of ATP.
Enzyme Activity and Regulation: Enzymes And Cellular Regulation Pogil
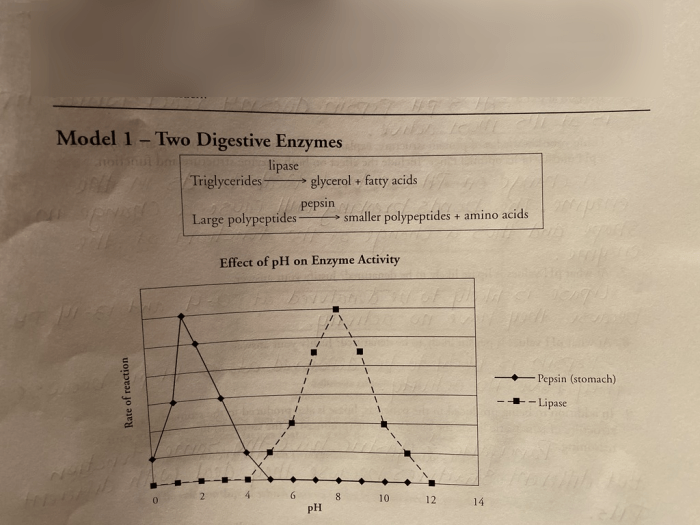
Enzyme activity is influenced by various factors, including temperature, pH, and substrate concentration. These factors affect the enzyme’s structure and its ability to bind to and catalyze the substrate.
Temperature
- Enzymes have an optimal temperature range at which they exhibit maximum activity.
- Deviations from this range can lead to decreased enzyme activity due to protein denaturation.
- Extreme temperatures can irreversibly denature enzymes, rendering them inactive.
pH
- Enzymes have an optimal pH range at which they are most active.
- Changes in pH can alter the ionization state of the enzyme’s active site, affecting its ability to bind to the substrate.
- Extreme pH values can denature enzymes, leading to loss of activity.
Substrate Concentration
- The rate of enzyme-catalyzed reactions increases with increasing substrate concentration.
- At low substrate concentrations, the enzyme is not saturated with substrate, and the reaction rate is proportional to the substrate concentration.
- As the substrate concentration increases, the enzyme becomes saturated, and the reaction rate reaches a maximum.
Enzyme Inhibition
Enzyme inhibition is a process that reduces the activity of an enzyme. There are two main types of enzyme inhibition: competitive and non-competitive.
Competitive Inhibition
- Competitive inhibitors bind to the enzyme’s active site, competing with the substrate for binding.
- The presence of a competitive inhibitor increases the apparent Km of the enzyme, indicating a decrease in the enzyme’s affinity for the substrate.
- Competitive inhibition can be overcome by increasing the substrate concentration.
Non-Competitive Inhibition
- Non-competitive inhibitors bind to the enzyme at a site other than the active site.
- They cause a conformational change in the enzyme, altering its catalytic activity.
- Non-competitive inhibition cannot be overcome by increasing the substrate concentration.
Enzyme Regulation
Enzyme regulation is crucial for controlling cellular metabolism. It ensures that metabolic pathways are coordinated and that the cell responds appropriately to changes in its environment.
There are various mechanisms of enzyme regulation, including:
- Feedback inhibition: The end product of a metabolic pathway inhibits the enzyme that catalyzes the first step in the pathway.
- Allosteric regulation: Small molecules bind to the enzyme at specific sites, altering its activity.
- Covalent modification: Enzymes can be modified by covalent attachment of groups such as phosphate or acetyl groups, affecting their activity.
Enzyme regulation plays a vital role in maintaining cellular homeostasis and ensuring efficient and coordinated cellular processes.
Case Study: Allosteric Regulation
Allosteric regulation is a mechanism of enzyme regulation where a molecule, known as an allosteric effector, binds to a specific site on the enzyme, causing a conformational change that alters the enzyme’s activity.
Role of Allosteric Effectors
Allosteric effectors can be either positive or negative, meaning they can either increase or decrease the enzyme’s activity, respectively. Positive allosteric effectors stabilize the active conformation of the enzyme, while negative allosteric effectors stabilize the inactive conformation.
Comparison of Positive and Negative Allosteric Regulation
| Positive Allosteric Regulation | Negative Allosteric Regulation | |
|---|---|---|
| Effect on enzyme activity | Increases enzyme activity | Decreases enzyme activity |
| Conformation of enzyme | Stabilizes active conformation | Stabilizes inactive conformation |
| Effect on substrate binding | Increases substrate binding | Decreases substrate binding |
| Example | Aspartate transcarbamoylase | Glyceraldehyde-3-phosphate dehydrogenase |
Enzyme Kinetics

Enzyme kinetics studies the rates of enzyme-catalyzed reactions and the factors that affect them. Understanding enzyme kinetics is crucial for comprehending how enzymes function in biological systems.
Michaelis-Menten kinetics is a widely used model that describes the relationship between the reaction rate and substrate concentration. According to this model, the reaction rate initially increases with increasing substrate concentration, but eventually reaches a maximum rate when all enzyme active sites are saturated with substrate molecules.
Factors Affecting Enzyme Kinetics
Several factors can influence enzyme kinetics, including:
- Enzyme concentration:The reaction rate is directly proportional to the enzyme concentration. As more enzyme molecules are present, more substrate molecules can be converted into products.
- Substrate affinity:The affinity of an enzyme for its substrate is measured by the Michaelis constant (Km). A lower Km value indicates a higher affinity, meaning the enzyme binds to the substrate more tightly. Enzymes with a higher affinity for their substrates will have a lower Km and a higher catalytic efficiency.
- Temperature:Enzyme activity is optimal at a specific temperature. As the temperature increases, the reaction rate initially increases due to increased molecular motion. However, at very high temperatures, the enzyme can denature, leading to a decrease in activity.
- pH:The pH of the environment can affect the ionization state of the enzyme and its substrate. Changes in pH can alter the enzyme’s conformation and its ability to bind to the substrate.
Graphical Representation of Enzyme Kinetics
The Michaelis-Menten curve is a graphical representation of enzyme kinetics. It plots the reaction rate against the substrate concentration. The curve has a hyperbolic shape, with an initial linear portion where the reaction rate increases with increasing substrate concentration, and a plateau region where the reaction rate reaches its maximum value.
The Michaelis-Menten equation, which describes the relationship between the reaction rate, substrate concentration, and kinetic parameters, is as follows:
v = Vmax
[S] / (Km + [S])
where:
- v is the reaction rate
- Vmax is the maximum reaction rate
- [S] is the substrate concentration
- Km is the Michaelis constant
Experimental Techniques in Enzyme Analysis
Investigating enzyme activity is crucial for understanding their roles in cellular processes. Various experimental techniques provide valuable insights into enzyme behavior.
Spectrophotometry, Enzymes and cellular regulation pogil
Spectrophotometry measures the absorbance of light by a sample at specific wavelengths. This technique is commonly used to study enzyme activity by monitoring the change in absorbance of a substrate or product.
- Principle:Enzymes catalyze reactions that involve the absorption or release of light, altering the absorbance of the solution.
- Applications:
- Measuring enzyme activity quantitatively
- Determining enzyme kinetics and reaction rates
- Identifying enzyme inhibitors and activators
Chromatography
Chromatography separates molecules based on their physical and chemical properties. This technique is used to identify and quantify enzymes and their products.
- Principle:Enzymes and other molecules are separated as they pass through a stationary phase, with different molecules interacting with the phase to varying degrees.
- Applications:
- Purifying enzymes for further study
- Separating and identifying enzyme isoforms
- Analyzing enzyme reaction products
Enzyme Assays
Enzyme assays are specific experiments designed to measure the activity of an enzyme. They involve measuring the rate of a reaction catalyzed by the enzyme under controlled conditions.
- Principle:Enzyme assays use a substrate specific to the enzyme, and the rate of substrate conversion is measured.
- Applications:
- Determining enzyme concentration
- Studying enzyme kinetics and reaction mechanisms
- Diagnosing enzyme deficiencies or abnormalities
Designing an Enzyme Activity Experiment
To measure the activity of a specific enzyme, an experiment can be designed using the following steps:
- Choose a suitable substrate:Select a substrate that is specific to the enzyme being studied.
- Establish assay conditions:Optimize pH, temperature, and other factors that affect enzyme activity.
- Prepare enzyme and substrate solutions:Prepare solutions of the enzyme and substrate at known concentrations.
- Initiate the reaction:Mix the enzyme and substrate solutions and incubate at the appropriate conditions.
- Measure reaction rate:Monitor the reaction progress using spectrophotometry, chromatography, or other methods.
- Calculate enzyme activity:Use the reaction rate data to calculate the enzyme activity in units of enzyme concentration or specific activity.
Question & Answer Hub
What is the role of enzymes in cellular processes?
Enzymes act as catalysts, accelerating biochemical reactions and enabling cellular processes to occur at a faster rate.
How are enzymes regulated?
Enzyme activity can be regulated through various mechanisms, including feedback inhibition, allosteric regulation, and covalent modification.
What is the significance of allosteric regulation?
Allosteric regulation allows enzymes to respond to changes in the cellular environment, ensuring metabolic pathways are coordinated and responsive to cellular needs.